
When growing up, I started collecting beer cans. It was fun discovering the unfamiliar beer cans found worldwide—their designs, what information brewers thought most important to share, and the shapes of the cans.
 It also provided my dad with amusement. Whenever my brother, dad, and I would go bird hunting, I’d invariably spend an impressive deal of time looking for junk piles people left in the woods. Dad, a big-time outdoorsman, spent time with me in the piles looking for cans. The prize finds were the old cone top cans. I’ll always remember him setting the shotgun along a tree, squatting down, and yelling, “found one!” All my brother Dean did was shake his head, roll his eyes, and keep walking.
It also provided my dad with amusement. Whenever my brother, dad, and I would go bird hunting, I’d invariably spend an impressive deal of time looking for junk piles people left in the woods. Dad, a big-time outdoorsman, spent time with me in the piles looking for cans. The prize finds were the old cone top cans. I’ll always remember him setting the shotgun along a tree, squatting down, and yelling, “found one!” All my brother Dean did was shake his head, roll his eyes, and keep walking.
As great as it was finding cone tops, it was also frustrating. The biggest reason? Rust, finding a can that didn’t have at least a light surface covered was uncommon. I researched and tried all methods of removing it but never found a suitable way. Remember, personal computers at that time were found only in sci-fi movies.
Seasons changed, and finding anything resembling an excellent catch became harder. Time doesn’t stand still, and neither does rust.
What Causes Rust?
Before solving a problem, you first need to understand it.
Rust results from a chemical reaction between iron, oxygen, and water. Although commonly referred to as iron oxide, rust is iron oxide hydrate because pure iron oxide isn't rust.
iron + water + oxygen = hydrated iron(III) oxide
Even though roughly 21% of air consists of oxygen, rust needs moist air and water. Oxidation doesn't occur in arid regions but thrives in other areas.
Because of the electrochemical nature of the reaction, dissolved electrolytes in water aid the reaction. Rust occurs more quickly in saltwater than in pure water, for example.
Keep in mind oxygen gas (O2) is not the only source of oxygen in air or water. Carbon dioxide (CO2) also contains oxygen. Carbon dioxide and water react to form weak carbonic acid. Carbonic acid is a better electrolyte than pure water. As the acid attacks the iron, water breaks into hydrogen and oxygen. Free oxygen and dissolved iron form iron oxide, releasing electrons, which can flow to another part of the metal. Once rusting starts, it continues to corrode the metal.
Most metals are susceptible to rusting. The only exceptions are rare metals such as gold, diamond, copper, and bronze. Rust occurs whenever exposing metals to natural elements such as rain, sunshine, and air. These elements bring with them water, heat, and oxygen—paramount for rust to occur. In other words, the absence of oxygen prevents rust.
Effects Of Rust On Metals
 Machinery is made from a combination of metallic substances with other materials such as plastic and wood. The metallic component of machines is most likely to rust in their everyday use if left unprotected. So then, what are the effects of rust on your equipment?
Machinery is made from a combination of metallic substances with other materials such as plastic and wood. The metallic component of machines is most likely to rust in their everyday use if left unprotected. So then, what are the effects of rust on your equipment?
When corrosion occurs on a metallic surface, the top layer of the surface reacts with the oxygen forming rust. Oxidation is referred to as ferric oxide. Ferrum is the chemical name of iron. Rust does not constitute a harmonious layer on the metal’s surface; therefore, it quickly falls off or is blown away in powder form. As a result, it exposes a fresh new surface of the metal to oxygen and the other elements, and the cycle of rusting continues.
Ultimately, when corrosion occurs repetitively, it compromises the object's structural integrity. As a result, equipment becomes weaker and, therefore, cannot handle the same workloads as it used to. In cases where the rust is not uniform, fatigue stress occurs on the corroded parts on elements of your machinery. This means components may crack or fail at their weakest point caused by severe corrosion. Heavy machinery often undergoes total failure or sharp performance reduction because of this fatigue. Either way, it’s not pleasant news.
Weakest Link
When the metal reinforcing other structural material rusts, it compromises the machine’s structural integrity. In other words, the bond strength of the metal decreases because of the destruction or disruption of the bond. You can further visualize this destruction by imagining how the different metals expand and contract, thanks to their unique chemical properties.
 With agricultural machinery, Caterpillar and John Deere have, over the years, dominated the industry. A significant problem faced by agrarian machinery is rusting. Rusting occurs because of the constant exposure of equipment to the natural elements. Unfortunately, this exposure is unavoidable thanks to oxygen in the air, water, and soil.
With agricultural machinery, Caterpillar and John Deere have, over the years, dominated the industry. A significant problem faced by agrarian machinery is rusting. Rusting occurs because of the constant exposure of equipment to the natural elements. Unfortunately, this exposure is unavoidable thanks to oxygen in the air, water, and soil.
The presence of rust in these pieces of machinery has adverse effects, such as contamination of agricultural products. If undetected, the ferric oxide, especially when in powder form, easily mixes with food substances, causing potential harm to all consumers.
Rusted machinery also puts workers and handlers at grave risk, as these machines could fail at any point during operation. Another prominent effect of oxidization in metals is the deterioration of the material’s appearance, making the equipment unsightly. Environmentally, there is no biodegradable way to dispose of unwanted iron oxide. This emphasizes the importance of avoiding rust at all costs.
Preventing Rust
Since it is impossible to isolate your agricultural machinery from the elements, the secret to preventing rust is by creating a shield between the metal and oxygen. A simple method of achieving this is by using metallic material resistant to rust. An excellent example is coating iron with chromium. Chromium is a non-corrosive alloy resistant to rust.
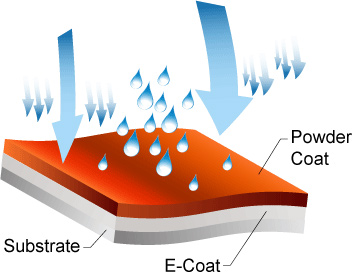 Another way to protect metal is by applying a protective barrier between the metal and elements using e-coating. Electrophoretic deposition (e coating/electric coating) is like electroplating. The electroplating process uses electric current reducing dissolved metal cations, forming a thin, coherent metal coating.
Another way to protect metal is by applying a protective barrier between the metal and elements using e-coating. Electrophoretic deposition (e coating/electric coating) is like electroplating. The electroplating process uses electric current reducing dissolved metal cations, forming a thin, coherent metal coating.
E coating occurs while dipping the parts in a bath of epoxy, water-based solutions, or paint while adding an electric current.
The current causes a reaction within the bath, depositing floating particles on the component. The amount of time using the electric current determines the coating thickness. After coating the part, it's moved to a curing oven finishing the process.
A common alternative is galvanization, which refers to zinc-plating your metal. Similar to chromium, zinc is also resistant to rust. Coating the metal with paint, lacquer, or even varnish is a more affordable option. However, this option requires frequent maintenance because of the “light” nature of the coating.
 Other methods of rust prevention that may not be beneficial in the agricultural sector include minimizing exposure of your machines to the natural elements and sacrificial coating. The latter primarily refers to coating your machine with an additional metal type that’s more likely to oxidize than the primary metal, hence the name.
Other methods of rust prevention that may not be beneficial in the agricultural sector include minimizing exposure of your machines to the natural elements and sacrificial coating. The latter primarily refers to coating your machine with an additional metal type that’s more likely to oxidize than the primary metal, hence the name.
In Sum
Neat as they were on the wall alongside other cans, rust took away some of the glamors of those cone-top beer cans. It was as frustrating as it was seeing them, looking at history, and spending time with my dad, but it was still pretty cool.
Rust is a natural process that occurs with or without our help. However, the only remaining option is finding smart ways to work around it. So if you have an old derelict piece of machinery that succumbed to corrosion, pick it up, scrape off the rust, identify the right preventive measure suitable for you, and get to work.